New scientific article!
Monitoring of monoclonal antibody critical quality attributes and free amino acids in CHO bioprocesses using Raman spectroscopy and DUPLEX-based PLS modeling.
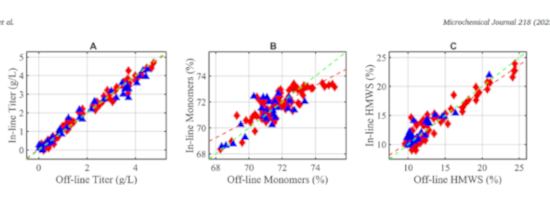
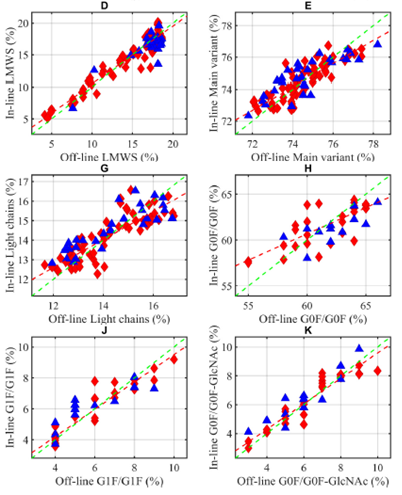
Published in Microchemical Journal in late September, this article presents work carried out on the online monitoring of Critical Quality Attributes (CQAs) of monoclonal antibodies (mAbs) and free amino acids in CHO cultures using Raman spectroscopy.
The PLS (Partial Least Square) modeling made it possible the prediction of the final quality criteria, the following CQAs: :
- Aggregates – multimeric monoclonal antibodies which affect stability and immunogenicity.
- Fragments – degradation or cleavage products of the mAb, impacting purity and efficiency.
- Glycoforms – variations in glycosylation profile, influencing pharmacokinetics and bioactivity.
- Charged variants – isoforms resulting from post-translational modifications (e.g., deamidation, amidation, C-terminal lysine), which affect stability and therapeutic performance.
🧪 Moreover, the developed models have also made it possible to predict free amino acids in the culture medium (dynamic nutritional profile), which allows simultaneous monitoring of cellular metabolism and product quality.
Morandise Rubini conducted this research as part of the CLIMBIN project with various researchers from the University of Tours, Indatech-Chauvin Arnoux Group, Servier Technology, and Ondalys. Jordane Poulain and Sylvie Roussel, co-authors of this article, contributed their expertise in Machine Learning.
Morandise Rubini, Anaïs Berger, Thomas Saillard, Fabrice Cantais, Martin Soucé, Jordane Poulain, Sylvie Roussel, Julien Louet, Fabien Chauchard-Rios, Sylvain Arnould, Igor Chourpa. (2025). Monitoring of monoclonal antibody critical quality attributes and free amino acids in CHO bioprocesses using Raman spectroscopy and DUPLEX-based PLS modeling. Microchemical Journal 218 (2025) 115583